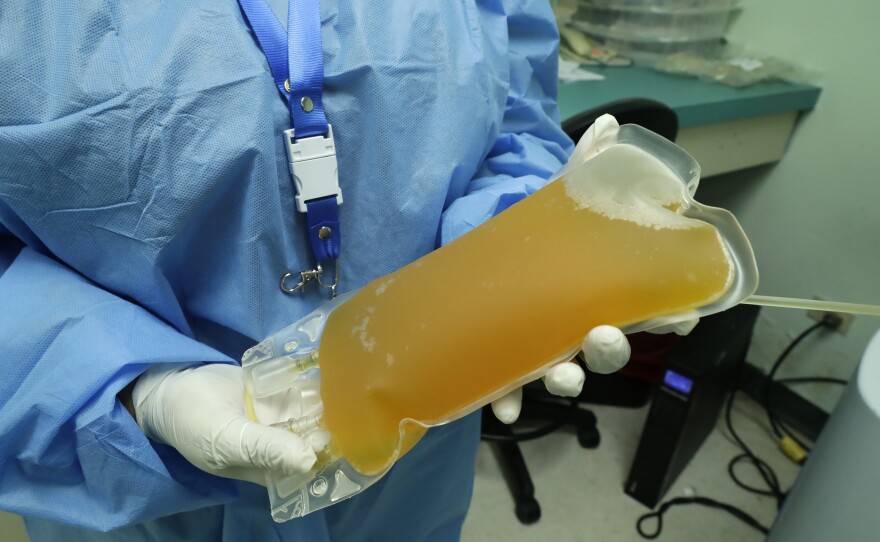
Doctors across San Diego have used convalescent plasma to treat hundreds of COVID-19 patients.
Demand for the therapy is expected to surge after a controversial move by the Food and Drug Administration. The FDA recently announced that convalescent plasma — the liquid part of blood that contains antibodies from donors who have recovered from an illness — can be used as an emergency treatment for COVID-19.
But it's effectiveness against the virus isn't clear. This week, the National Institute of Health said there's no evidence to support the use of plasma for the treatment of COVID-19.
RELATED: Trump Wanted FDA To Fast-Track Blood Plasma Therapy. What Is It?
Reporter Jonathan Wosen, who covers the biotech industry for The San Diego Union-Tribune, has looked into plasma therapy and joined Midday Edition on Tuesday to share his reporting.